- Coronary Stents Coronary Balloons Catheters Guide Wires Introducer Sheaths Fractional Flow Reserve Accessories Peripheral Interventional Products
- Pacemakers and Leads Equipments
- Needle-free Connectors CVC Accessories Blood Pressure Transducer Arterial Blood Sampler IV Catheter Insulin Injection Pain Relief Vascular Access
- Hemodialysis consumables Hemoperfutor
- POCT IMMUNOASSAY MOLECULAR DIAGNOSTICS HEMOTALOGY DIAGNOSTICS Others
- ECG Patient Monitor Oximeter
- Cardiovasculars Oncology Anti-infectives Central Nervous System Others
- Surgical Staplers Endoscopy Endoscopic Ultrasound System
- Structural Heart Diseases Products
- Trauma Implant Spinal Implant Joint System Surgical Instruments
- Colloidal Gold Quantitative Analyzer SARS-CoV-2 Test Kits 2019-nCoV Neutralization Antibody Test Kit Poctor® blood glucose, ketone body, uric acid detector Cholesterol Analyzer PT/INR Meter Immunofluorescence Quantitative Analyzer
- Fully Automated ELISA workstation Fully Automated CLIA workstation Automatic fluorescence immunoassay analyzer
- Fully Automated Nucleic Acid Extractor Nucleic Acid Extraction or Purification Kit Real-Time PCR System
- Thromboelastography Analyzer Fully automated blood grouping system
- Other Platforms
- NeoECG Holter Monitor Portable ECG Monitor OmniECG AI-ECG Pocket ECG
- Patient Monitor Vital Signs Monitor End-tidal Capnography
- Fingertip Oximeter Handheld Oximeter Wrist Oximeter Wearable Oximeter All-in-One Health Monitor POCT Solution
- Locking Compression Plates System Locking Plates Screws Interlocking Intramedullary Nails System Mini Plates System Conventional Plates Metal Pins Dynamic Hip Locking Plate
- Anterior Cervical Locking Fixation System Laminoplasty Plate Fixation System Internal Spinal Fixation System Spinal Fusion System
- Instrument Set of Arthroplasty System
- Guide Wires
- Introducer Sheaths
- Fractional Flow Reserve
- Accessories
- Peripheral Interventional Products

MemoPart™ Patent Ductus Arteriosus (PDA) Occluder


MemoPart™ Patent Ductus Arteriosus (PDA) Occluder Details
Product Description
MemoPart™ Patent Ductus Arteriosus (PDA) Occluder is a self-expandable device made from nitinol wire mesh. Aretention skirt on the aortic side provides secure positioning in the ampulla of the ductus. As the occluder is implanted, it expands outward and the wires push against the wall of the ductus. Polyester fabric is sewn into the occluder with polyester thread.The fabric induces thrombosis that closes the communication.
Product Features
Modified rigidity and flexibility for different type of occluders - balanced stiffness of Patent Ductus Arteriosus Occluder (PDAO) ensures the optimal occlusion performance.
Uniform and smooth oxide film on nitinol wire effectively prevents from the release of nickel ion to guarantee the great biocompatibility and long-term safety.
The hubless design of Patent Ductus Arteriosus Occluder (PDAO) helps with earlier endothelialization to minimize the risk of thrombosis.
Indications for Use
The MemoPart™ Patent Ductus Arteriosus (PDA) Occluder can be used for the nonsurgical closure of apatent ductus arteriosus (PDA) in the percutaneous, transcatheter therapy.
Ordering Information
Cone Shape Patent Ductus Arteriosus (PDA) Occluder
| Catalogue No. | Device Size | B Aortic Waist Diameter (mm) | C Pulmonic Waist Diameter (mm) | A Aortic Disc Diameter (mm) | H Waist Length (mm) |
| WBFDQ- Ⅱ 06 | 406 | 6 | 4 | 10 | 6 |
| WBFDQ- Ⅱ 08 | 608 | 8 | 6 | 12 | 6.5 |
| WBFDQ- Ⅱ 10 | 810 | 10 | 8 | 14 | 7.5 |
| WBFDQ- Ⅱ 12 | 1012 | 12 | 10 | 16 | 8.5 |
| WBFDQ- Ⅱ 14 | 1214 | 14 | 12 | 18 | 9.5 |
| WBFDQ- Ⅱ 16 | 1416 | 16 | 14 | 20 | 10.5 |
| WBFDQ- Ⅱ 18 | 1618 | 18 | 16 | 23 | 10.5 |
| WBFDQ- Ⅱ 20 | 1820 | 20 | 18 | 25 | 12 |
| WBFDQ- Ⅱ 22 | 2022 | 22 | 20 | 27 | 12 |
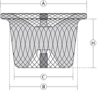
Hubless Cone Shape Patent Ductus Arteriosus (PDA) Occluder
| Catalogue No. | Device Size | B Aortic Waist Diameter (mm) | C Pulmonic Waist Diameter (mm) | A Aortic Disc Diameter (mm) | H Waist Length (mm) |
| WTWBFDQ- Ⅱ 06 | 406 | 6 | 4 | 10 | 6 |
| WTWBFDQ- Ⅱ 08 | 608 | 8 | 6 | 12 | 6.5 |
| WTWBFDQ- Ⅱ 10 | 810 | 10 | 8 | 14 | 7.5 |
| WTWBFDQ- Ⅱ 12 | 1012 | 12 | 10 | 16 | 8.5 |
| WTWBFDQ- Ⅱ 14 | 1214 | 14 | 12 | 18 | 9.5 |
| WTWBFDQ- Ⅱ 16 | 1416 | 16 | 14 | 20 | 10.5 |
| WTWBFDQ- Ⅱ 18 | 1618 | 18 | 16 | 23 | 10.5 |
| WTWBFDQ- Ⅱ 20 | 1820 | 20 | 18 | 25 | 12 |
| WTWBFDQ- Ⅱ 22 | 2022 | 22 | 20 | 27 | 12 |
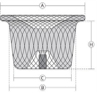
Cylinder Shape Patent Ductus Arteriosus (PDA) Occluder
| Catalogue No. | Device Size | B Aortic Waist Diameter (mm) | C Pulmonic Waist Diameter (mm) | A Aortic Disc Diameter (mm) | H Waist Length (mm) |
| WBFDQ- Ⅰ 04 | 4 | 4 | 4 | 8 | 4.0 |
| WBFDQ- Ⅰ 05 | 5 | 5 | 5 | 9 | 5.0 |
| WBFDQ- Ⅰ 06 | 6 | 6 | 6 | 10 | 6.0 |
| WBFDQ- Ⅰ 07 | 7 | 7 | 7 | 11 | 6.5 |
| WBFDQ- Ⅰ 08 | 8 | 8 | 8 | 12 | 6.5 |
| WBFDQ- Ⅰ 09 | 9 | 9 | 9 | 13 | 7 |
| WBFDQ- Ⅰ 10 | 10 | 10 | 10 | 14 | 7.5 |
| WBFDQ- Ⅰ 11 | 11 | 11 | 11 | 15 | 8 |
| WBFDQ- Ⅰ 12 | 12 | 12 | 12 | 16 | 8.5 |
| WBFDQ- Ⅰ 13 | 13 | 13 | 13 | 17 | 8.5 |
| WBFDQ- Ⅰ 14 | 14 | 14 | 14 | 18 | 9.5 |
| WBFDQ- Ⅰ 16 | 16 | 16 | 16 | 21 | 10.5 |
| WBFDQ- Ⅰ 18 | 18 | 18 | 18 | 23 | 10.5 |
| WBFDQ- Ⅰ 20 | 20 | 20 | 20 | 25 | 12 |
| WBFDQ- Ⅰ 22 | 22 | 22 | 22 | 27 | 12 |
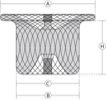
Hubless Cylinder Shape Patent Ductus Arteriosus (PDA) Occluder
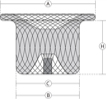
| Catalogue No. | Device Size | B Aortic Waist Diameter (mm) | C Pulmonic Waist Diameter (mm) | A Aortic Disc Diameter (mm) | H Waist Length (mm) |
| WTWBFDQ- Ⅰ 04 | 4 | 4 | 4 | 8 | 4 |
| WTWBFDQ- Ⅰ 05 | 5 | 5 | 5 | 9 | 5 |
| WTWBFDQ- Ⅰ 06 | 6 | 6 | 6 | 10 | 6 |
| WTWBFDQ- Ⅰ 07 | 7 | 7 | 7 | 11 | 6.5 |
| WTWBFDQ- Ⅰ 08 | 8 | 8 | 8 | 12 | 6.5 |
| WTWBFDQ- Ⅰ 09 | 9 | 9 | 9 | 13 | 7 |
| WTWBFDQ- Ⅰ 10 | 10 | 10 | 10 | 14 | 7.5 |
| WTWBFDQ- Ⅰ 11 | 11 | 11 | 11 | 15 | 8 |
| WTWBFDQ- Ⅰ 12 | 12 | 12 | 12 | 16 | 8.5 |
| WTWBFDQ- Ⅰ 13 | 13 | 13 | 13 | 17 | 8.5 |
| WTWBFDQ- Ⅰ 14 | 14 | 14 | 14 | 18 | 9.5 |
| WTWBFDQ- Ⅰ 16 | 16 | 16 | 16 | 21 | 10.5 |
| WTWBFDQ- Ⅰ 18 | 18 | 18 | 18 | 23 | 10.5 |
| WTWBFDQ- Ⅰ 20 | 20 | 20 | 20 | 25 | 12 |
| WTWBFDQ- Ⅰ 22 | 22 | 22 | 22 | 27 | 12 |
Thank You for Your Attention on Lepu Medical!
Email us with any questions or inquiries or use our contact data. We would be happy to answer your questions.
